| ||||||||
|
| ||||||||
|
Tutorial: Chemical EquilibriumPart 5- Le Châtelier's Principle I- Concentration |
There are a number of movies available on this page. I've given you a choice of files in most cases since movies can be very large and take forever to download. If you have a good connection (you're at achool, ISDN, fast modem connection, etc.) then choose the "high res" file. If you have a slow connection then choose the "low res" file.
Now that we've discussed what chemical equilibrium means and we've done a number of different calculations, we can see how we can qualitatively affect equilibria. Le Châtelier's Principle states-
...if an outside influence upsets an equilibrium, the system undergoes a change in a direction that counteracts the disturbing influence and, if possible, returns the system to equilibrium.
We can "stress" a chemical reaction by applying some outside influence which takes the reaction out of equilibrium. This stress results in the system no longer being in equilibrium (Q ≠ Kc) and then a corresponding reaction occurs as the system returns to equilibrium (Q = Kc). This does not mean that all the concentrations, pressures, etc. are the same as before the stress was applied. The common mistake to make is for people to think that when the system returns to equilibrium it returns to the same equilibrium as before the stress was applied. There are three methods to stress a system that's in chemical equilibrium-
A nice and very illustrative way to see how a system responds to a stress is to watch color changes. I have a number of them for concentration and temperature changes. For the effect of a pressure change, I have something else that's very common and will allow me to dispel a commonly believed myth.
A solution of iron(III) nitrate and potassium thiocyanate is prepared and then split into three different tubes. The center test tube shows the initial color of all three of them.
![]()

|
The tube on the left is the result of adding more iron(III) ion (via Fe(NO3)3). Note the color change from red to dark red. The tube on the right is the result of removing iron(III) ion by adding sodium hydroxide and producing solid iron(III) hydroxide (the red specks). |
Let's look at the chemical equation again with some additional information about solution color-
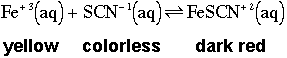
So, the initial solution color (middle tube) is a mixture of yellow and dark red colors due to the presence of both the Fe+3 ion and the FeSCN+2 complex. Reminder- to have an equilibrium you must have some of every species present. The addition of Fe+3 ion causes the solution to turn darker red and the removal of Fe+3 ion causes the solution to turn yellow (ignoring the solid iron(III) hydroxide suspended in solution).
What can we draw from all of that? Adding a chemical species to a system at equilibrium causes it to no longer be in equilibrium. Let's examine it by looking at the reaction quotient equation for this reaction.
![]()
OK. The addition of Fe+3 ion increases its concentration and thus makes the reaction quotient, Qc, smaller than Kc. For the system to once again be in equilibrium, Qc must increase until it equals Kc. Therefore, the reaction shifts towards the products (or, to the right) and the concentration of the FeSCN+2 complex increases which causes the solution color to become darker red.
Similar idea for the removal of Fe+3 ion. This decreases its concentration and thus increases Q. To regain equilibrium, Q must decrease until it equals Kc. The reaction shifts towards the reactants (or, to the left) and the concentration of both the Fe+3 ion and the SCN- complex increases. Since the Fe+3 ion is the only one of the two that is colored (yellow), the system changes to a yellow color.
Let's summarize-
This next reaction is very cool and extraordinarily complicated. So much so that I'm not going to put up the chemical equations! All three of the starting components are colorless-



Upon mixing them together, the solution immediately turns light orange and then begins to do something interesting. This reaction has two pathways and the concentrations are constantly changing which causes the interesting part.
I've looped only two oscillation cycles to keep the video size from being too large. This reaction will continue to oscillate between colorless-orange-dark blue/black for 10 minutes or longer. If you are curious about it and wish to dig up more info, search on the "Briggs-Rauscher Oscillating Reaction".
And here's another one that has a neat twist. It's sometimes referred to as the "blue bottle" trick in magic shows.
The flask is stoppered the entire time and all I'm doing is shaking the flask. The agitation causes the chemical indicator methlyene blue to react with oxygen and turn blue. While the vessel sits, the oxygen that's immediately available to react is used up and this causes the solution to turn colorless. This is used in biological applications when testing for anaerobic activity.
![]()
![]()
![]() updated 7:10 PM, 6/30/02
updated 7:10 PM, 6/30/02
chemistry@chemistry.alanearhart.org
© Copyright Notice