| ||||||||
|
| ||||||||
|
Tutorial: Chemical EquilibriumPart 6- Le Châtelier's Principle II- Temperature |
There are a number of movies available on this page. I've given you a choice of files in most cases since movies can be very large and take forever to download. If you have a good connection (you're at achool, ISDN, fast modem connection, etc.) then choose the "high res" file. If you have a slow connection then choose the "low res" file.
Let's restate Le Châtelier's Principle-
...if an outside influence upsets an equilibrium, the system undergoes a change in a direction that counteracts the disturbing influence and, if possible, returns the system to equilibrium.
and three methods to stress a system that's in chemical equilibrium-
The following reaction shows distinct color changes when the original solution is heated and when it is cooled.
![]()
|
The picture on the left shows the color of the solution at room temperature and the image on the right shows the color of the solution at either low temperature or high temperature. Hit the "swap" button to see the color change. Additionally, the changing image was the same solution. In other words, I first cooled it down to get the one color. I then heated the same solution up to see if the color would change again. This shows that chemical equilibrium can be reversible. |
You can see in the 5oC color that it's split between pink in the portion of the test tube in the cold water bath and the original purplish color in the portion of the tube that's not in the cold water bath. Let's take a look at the chemical equation with some solution color information added.
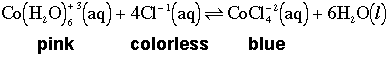
I didn't add any chemicals to shift the equilibrium but I did add heat by raising the temeprature and I did remove heat by lowering the temperature. The temperature effect upon equilibrium can be explained much like the way we did it for changes in concentration(s). Let's take one more look at the chemical equation but let's add in heat to one side or the other like we did for thermodynamics. But to which side do we add heat? To the reactants side or the products side? You have enough information to figure it out. Click on the equation you think is the correct one.
Make sure you try the above exercise before moving on!
Hopefully you can see what I mean when I say we can treat temperature changes like concentration changes. Raising the temperature is the same as adding heat and lowering the temperature is the same as removing heat. So, let's summarize-
![]()
![]()
![]() updated 7:04 PM, 6/30/02
updated 7:04 PM, 6/30/02
chemistry@chemistry.alanearhart.org
© Copyright Notice