
|
|
Jump back to one of the following links:
|
|
|
|

|
|
|
Jump to one of the following gas laws' parts:
|
| |
|
|
|
What about inflating a balloon without blowing it up? I've placed a partially
inflated balloon into a Bell jar. While you can't see details on the pressure gauge
in the upper-right portion of the video, you can see it change. As the needle
moves counter-clockwise, it shows the pressure decreasing in the jar. The movie
will cycle back-and-forth so you can watch it expand and contract.
This shows an application of Boyle's law. The amount of air (gas) in the balloon
is constant and all I'm doing is changing the external pressure on the balloon. A
sudden decrease in external pressure causes the volume of the balloon to increase
and the internal air pressure to decrease until the pressures match and the size of the
balloon is again constant. For the record, I made the time-lapsed video by snapping
pictures of the balloon's volume decreasing as I allowed the external pressure to
increase. I then assembled the pictures in reverse order since I wanted to show the
balloon initially expanding.
These quizzes will give you a chance to solve some basic gas law problems using
Boyle's, Gay-Lussac's, Charles', the Combined, and the Ideal gas laws. You need the two
Gas Constants-
The quiz will prompt you about the units for the answer and when you hit the "Grade Me!" button, it will check your answer for the proper number of significant
figures and warn you not only if your answer is wrong but if you have the wrong
number of significant figures. If your answer is correct or if you can't get the correct
answer and hit the "Reveal!" button, the formulas along with the solution set-up
(and cancelled units) will be displayed in the window.
Due to limitations with the display of math in browsers (although that is
changing very quickly... thankfully!) and the frustration with using browser
plugins (don't ask!), mathematical equations don't display exactly the way I'd
like them to. The cancellation of units doesn't look quite like I wish it would,
either. Here's the way it looks in two different browsers-

|
or
|

|
I'd rather the formatting of the equations look more like the following one
(minus the unit cancellation).


 updated 6:55 PM, 6/30/02
updated 6:55 PM, 6/30/02
chemistry@chemistry.alanearhart.org
© Copyright Notice


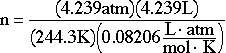
![]()
![]()
![]() updated 6:55 PM, 6/30/02
updated 6:55 PM, 6/30/02